Part:BBa_K3007003
Expression device of HucR & YgfU: Uric acid detector & uric acid transporter
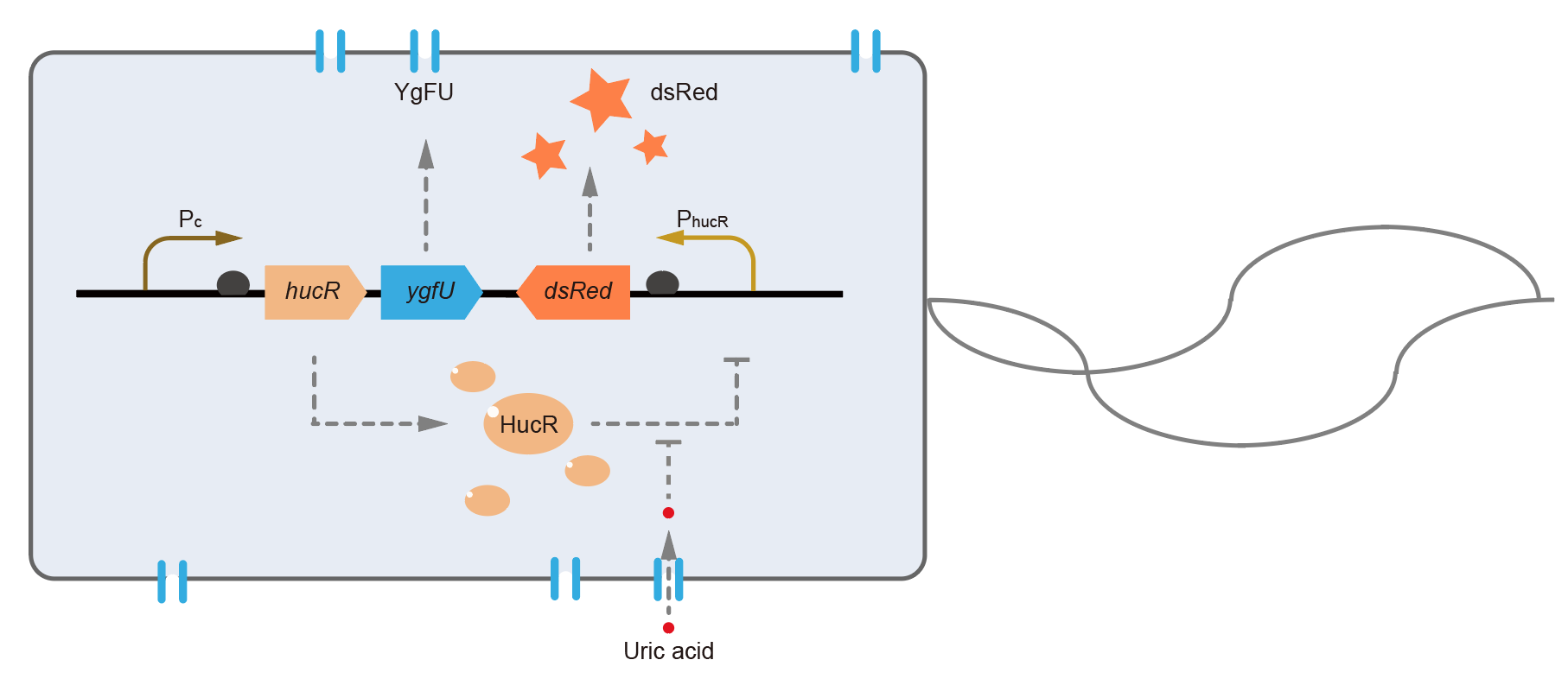
Hypothetical uricase regulator (HucR) from D. radiodurans is a bacterial transcriptional factor, which belongs to the MarR family. It acts as a homo-dimer and binds to a special dyad-symmetrical operator site named hucO in the absence of uric acid with high affinity (Kd = 0.29 ± 0.02 nM) [1]. When uric acid is present, HucR dissociates from hucO, allowing expression of a downstream gene. So, it can be adapted to develop a uric-acid-responsive regulatory system.
However, the cell wall and outer membrane of E. coli are barriers which cause the concentration of intracellular uric acid may be different from it outside the cell. That means the HucR-hucO system inside the cell may not react to accurate uric acid concentration in environment. To solve this problem, we find a protein named YgfU, which is a proton-gradient dependent, low-affinity (Km = 0.5 mM), and high-capacity (Vmax = 715 nmol·min-1·mg-1) transporter for importing uric acid into cytoplasm in E. coli [2].
In order to simplify the experiment design, we added the sequence coding YgfU after the CDS of HucR under the control of a continuous promoter, Pcp6 promoter, which forms a new composite part to transport and detect the exocellular uric acid.
Contribution
Group: QHFZ-China iGEM 2019
Author: Cheng Li
Design:
Documentation:
This year, QHFZ-China designed a UA monitor system in E. coli (Fig. 1). Pc is a constitutive promoter, Pcp6 promoter, and it promotes the expression of HucR and YgfU. If the concentration of uric acid (UA) in environment is low, HucR will bind to hucO sequence (located in PhucR), which inhibits the expression of downstream reporter, dsRed or sfGFP. When extracellular UA is present, YgfU can transport UA into the cytoplasm, which leads HucR dissociates from hucO, and induces the fluorescent protein expression.
We used the process shown in Fig. 2 to test if the UA detection system works well.
Two clones with UA detection system with dsRed as a reporter were tested. The original gene circuit was able to response to UA in a range of 0 to 200 μM (Fig. 3A), and the clone 1 showed much better dynamics than the other (Fig. 3B). Time course experiments showed that the fluorescence intensity became quite strong at 4 to 6 hours after UA induction, and became stable at 10 to 12 hours (Fig. 3C). Even if we removed UA by replacing fresh LB medium, after 48 hours shaking, the fluorescence would be still notable (Fig. 3D) and there was not significant difference of dsRed fluorescence / OD600 between before and after UA removing (Fig. 3E). All the data meant our design could detect high UA concentration quickly and stably.

We also tried more conditions to test if this system could work well in different environment. In the range of pH 6.0 to 8.0, response of the gene circuit was relatively stable (Fig. 4A). However, the volume of the reaction system would influence the response to UA (Fig. 4B). A possible explain was the relative surface area of the liquid level changed and consequently the dissolved oxygen changed. This result meant the experiments for UA detection should be done at the same reaction system volume. In other experiments, 1 mL reaction volume was used.

Because the designed applications included to detect UA in blood or saliva sample, which contained serum, we tested if serum affects the detection efficiency. In view of safety, commercial fetal bovine serum (FBS) (EVERY GREEN, 11011-8611) was used here. The growth of the bacteria was obviously suppressed when the volume of FBS fraction was more than 1/1000 (Fig. 5A, 5B), which meant 1 μL fetal bovine serum was added to 1000 μL final reaction system. When the volume of FBS fraction was 1/1000, the UA detection efficacy was unaffected by serum (Fig. 5C).

We verified the designed system did response UA in different environments. However, during our human practices, some of interviewees worried about long responding time, which needed 4 to 6 hours after UA induction to express strong fluorescence intensity. In their opinions, users would not wait for a 4-hour reaction. And there was another feedback that the standard of hyperuricemia is ≥7 mg/dL (about 400 μM) UA for men and ≥6.0 mg/dL (about 350 μM) UA for women [3]. If we want to use our system to detect a clinical sample directly, the sample should be diluted to 1/1000 before start, which means the gene circuit is required to detect 400× 1/1000 = 0.4 μM UA as a threshold. In one word, we need a modification to shorten the responding time and increase the sensitivity of the UA detector.
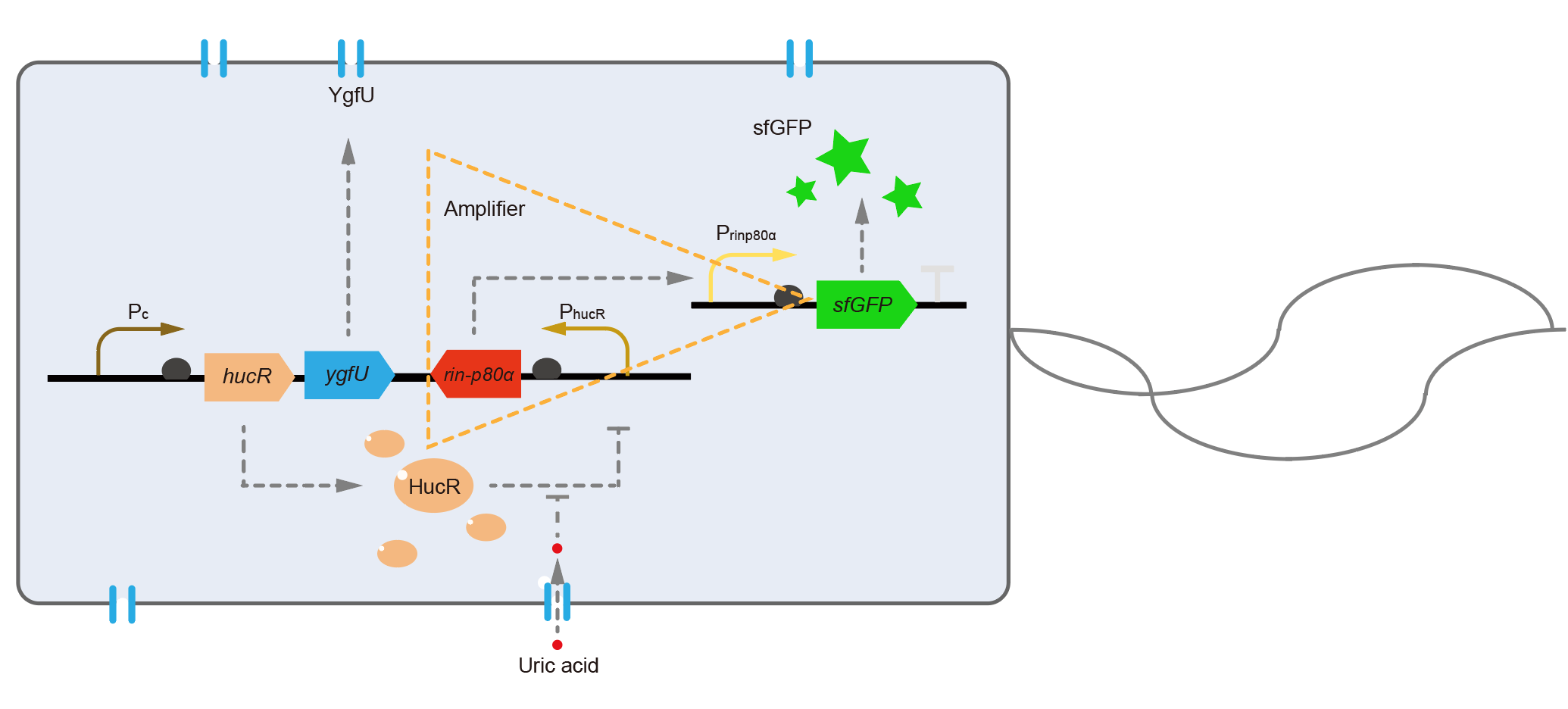
For the first question, we interviewed Dr. Xiaoyu Chen, a scientist who majors in biosensors. He suggested us that changing the fluorescent protein was a means to optimize the response speed. We blasted the sequence of dsRed we used in the database, and found the maturation half-time of dsRed is about 40 minutes in E. coli [4]. To shorten the maturation time, we decided to change dsRed to superfolder GFP, whose maturation half-time was only about 13 minutes [5]. To solve the second problem, we referenced a modular, cascaded signal amplifying methodology, which induces a module named amplifier, and it may increase sensitivity of the biosensor and boost the output expression [6]. We ordered two sequences of ultrasensitive phage activator RinA_p80α (from Staphylococcal aureus phage 80α) and a promoter PrinA_p80α. We introduced these new parts to design a new version of the UA detection system, called Version 2, shown in Fig. 6.
The processes in Version 2 were almost equal to the old version, except that the downstream of PhucR was RinA_p80α. This meant if UA presented, RinA_p80α would express and active transcription of sfGFP which was under control of PrinA_p80α. Theoretically, the new design would sense UA with much higher sensitivity than the old one. In the same time, the fluorescence production of Version 2 would get faster because that sfGFP had a shorter maturation time than dsRed in old version.
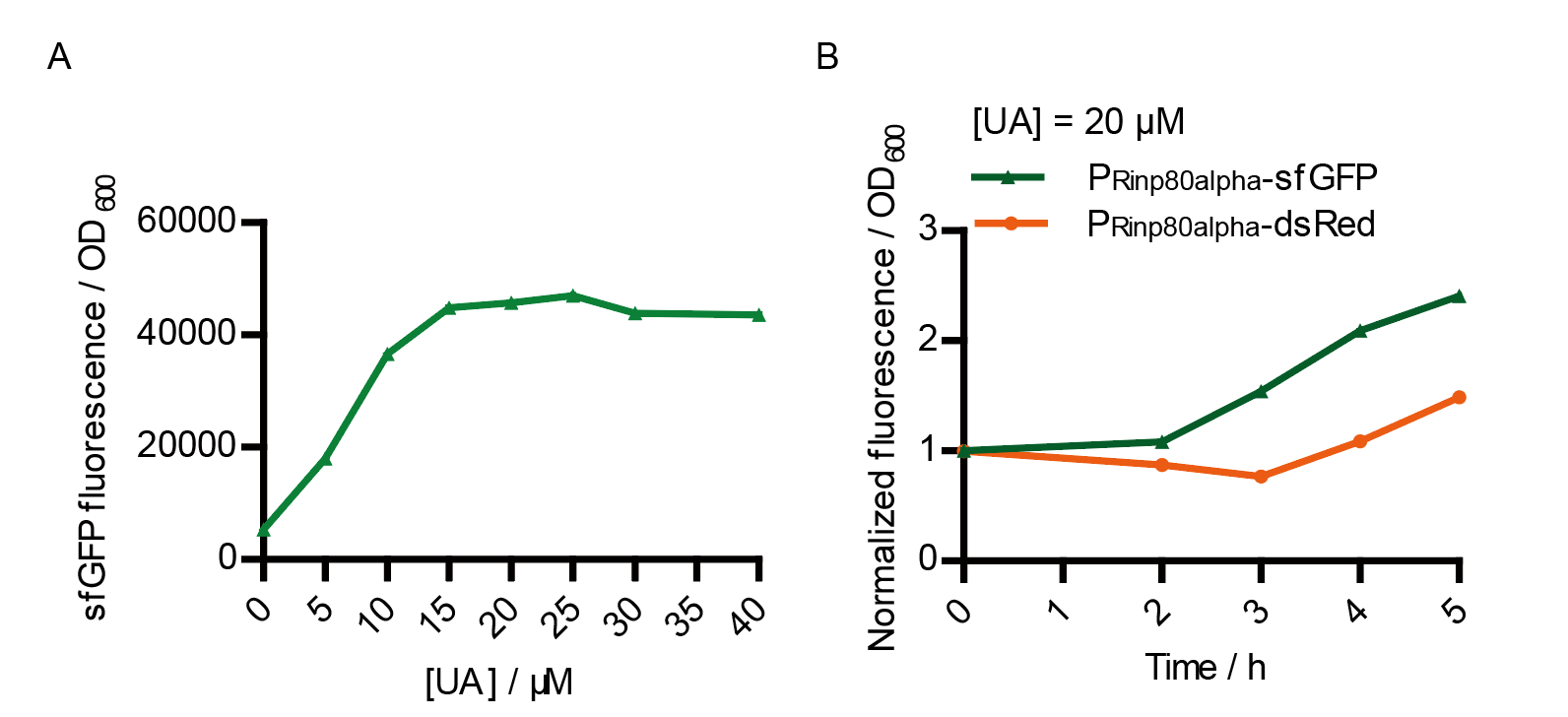
We tested the sfGFP production of Version 2 under different concentration of extracellular UA. The curve in Fig. 7A showed the fluorescence was saturated under only 15 μM UA induction, while the old version needed about 100 μM UA to get saturated (Fig. 3B). This result verified the Version 2 had higher sensitivity than the old one. To test if sfGFP could shorten the reaction time, we used the same construct only except reporter genes, called PRinA_p80α – sfGFP and PRinA_p80α – dsRed, respectively. After adding 20 μM UA into the reaction system, the curve of PRinA_p80α – sfGFP climbed much faster than PRinA_p80α – dsRed, which suggested our new design had a great induction performance, and fitted our predictions very well.
References:
[1] Liang C., Xiong D., Zhang Y., Mu S. and Tang S. (2015). Development of a novel uric-acid-responsive regulatory system in Escherichia coli. Appl. Microbiol. Biotechnol. 99, 2267–2275.
[2] Papakostas K. and Frillingos S. (2012). Substrate selectivity of YgfU, a uric acid transporter from Escherichia coli. J. Biol. Chem. 287:15684– 15695
[3] de Oliveira, E. P., & Burini, R. C. (2012). High plasma uric acid concentration: causes and consequences. Diabetology & metabolic syndrome, 4(1), 12.
[4] Bevis, B. J., & Glick, B. S. (2002). Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nature biotechnology, 20(1), 83.
[5] Pédelacq, J. D., Cabantous, S., Tran, T., Terwilliger, T. C., & Waldo, G. S. (2006). Engineering and characterization of a superfolder green fluorescent protein. Nature biotechnology, 24(1), 79.
[6] Wan, X., Volpetti, F., Petrova, E., French, C., Maerkl, S. J., & Wang, B. (2019). Cascaded amplifying circuits enable ultrasensitive cellular sensors for toxic metals. Nature chemical biology, 15(5), 540.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 32
Illegal EcoRI site found at 1849
Illegal XbaI site found at 135
Illegal PstI site found at 38 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 32
Illegal EcoRI site found at 1849
Illegal PstI site found at 38 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 32
Illegal EcoRI site found at 1849
Illegal BamHI site found at 43
Illegal BamHI site found at 118
Illegal BamHI site found at 982 - 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 32
Illegal EcoRI site found at 1849
Illegal XbaI site found at 135
Illegal PstI site found at 38 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 32
Illegal EcoRI site found at 1849
Illegal XbaI site found at 135
Illegal PstI site found at 38
Illegal AgeI site found at 1164 - 1000COMPATIBLE WITH RFC[1000]
| None |